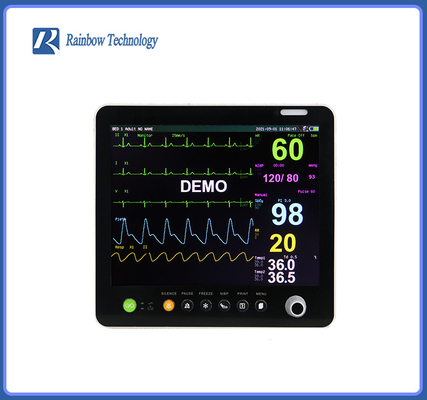
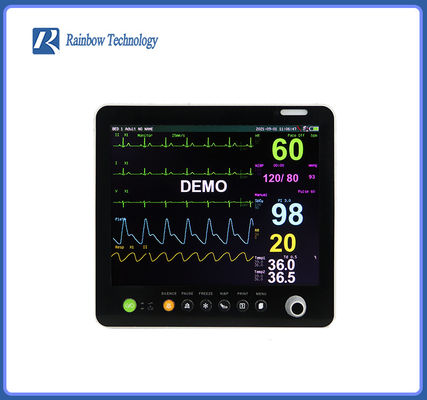
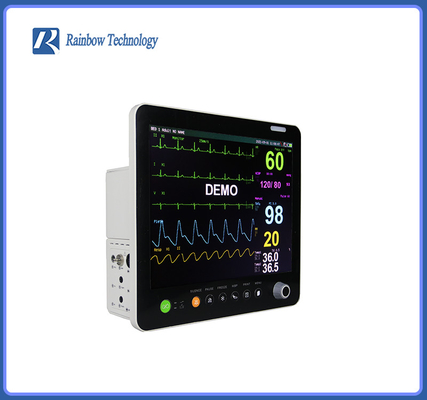
Touch Screen Global Hot Sale Professional After Service Pathological Analysis Bedside Monitor
PM9000-GTE Product Specification
Product brief
| 1. ARM9 main board + 7in1 monitor module; Based on embedded linux, the monitor have small size, high integration, low power and high performance |
| 2. Parameters include ECG, Respiration, NIBP, dual temperature,SpO2, pulse rate, dual IBP, CO₂ and 3. Multi-Gas |
| 4. Unique human voice alarm |
| 5. Full digital SpO2 technology with excellent performance in low perfusion and movement |
| 6. ECG monitoring include arrhythmia analysis, ST calculation and Pace analysis |
| 7. Protected against defibrillation, ESU and myoelectric artifact |
| 8. NIBP have dual over pressure protect, can operation correctly under severe environment to protect the patient |
| 9. Full alarm feature include 3 alarm level, alarm sound, alarm light and on screen alarm message |
| 10. Human voice alarm helps the user to identify the source of alarms |
| 11. Embedded network can connect to central monitor server |
| 12. Maximum 720 hours trend review, 1000 NIBP review, 2 hours full wave review and 200 alarm event review |
| 13. USB port support USB disk data output and PRS software to review patient data |
| 14. USB WIFI module support wireless central monitor |
| 15. 15’high resolution LCD, big font screen provided |
| 16. Optional touch screen for better user interface |


Specification
 Warning
Warning 
The patient monitor may not meet its performance specification if stored or used outside the manufacturer’s specified temperature and humidity range.
1.1 Classification
Anti-electroshock type Class I equipment
Anti-electroshock degree CF defibrillation
1.2 Accordant Standard
| Medical Device Directive 93/42/EEC |
|
| EN60601-1:2005 or IEC60601-1:2005 |
Medical Electrical Equipment, Part 1: General Requirements for Safety |
| EN60601-1-1 or IEC60601-1-1 |
Medical Electrical Equipment, Part 1-1: General Requirements for Safety, Collateral Standard: Safety Requirements for Medical Electrical Systems |
| IEC60601-1-4 |
Medical Electrical Equipment, Part 1-4: General Requirements for Safety, Collateral Standard: Programmable Electrical Medical Systems |
| IEC60601-2-49 |
Medical Electrical Equipment, Part 2-49: Particular Requirements for the Safety of Multifunction Patient Monitoring Equipment |
| IEC 60601-1-2:2007 |
Electromagnetic Compatibility, Medical Electrical Equipment |
| ISO 21647:2009 |
Medical electrical equipment — Particular requirements for the basic safety and essential performance of respiratory gas monitors |
1.3 Power Supply
100~240 VAC, 50/60 Hz, Pmax=60VA
1.4 Battery
2200 mAh 14.8V rechargeable Lithium battery
Operating time after full charge is more than 1.5 hours
Operating time after the first alarm of low battery will be about 5 minutes
Maximum charging time is less than 6 hours.
1.5 Environment
Temperature
Working 0 ~ 40 °C, 5 ~ 40 °C with CO₂
Storage -20 ~ 50°C
Humidity
Working 15% - 90 %
Storage 15% - 90 % (no coagulation)
1.6 Signal Interface
Network interface standard RJ45 Socket
USB port
1.7 ECG
1.7.1 Lead mode
5 Leads, I, II, III, AVR, AVL, AVF, V
1.7.2 Gain
´2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV
1.7.3 Heart rate
Measure range:
Adult 15 ~ 300 bpm
Neonatal/Pediatric 15 ~ 350 bpm
accuracy ± 1%
resolution 1 bpm
1.7.4 Sensitivity
> 200 μV P-P
1.7.5 Differential Input Impedance
> 5 M ohm
1.7.6 CMRR
Diagnostic Mode >90 dB
Monitor Mode >110 dB
Surgery Mode >110 dB
1.7.7 Electrode offset potential
±300mV
1.7.8 Leakage Current
< 10 μA
1.7.9 PACE pulse detect
range ±2~±700mV
width 0.1~2ms
rise time 10~100µs
1.7.10 PACE pulse rejection
range ±2~±700mV
width 0.1~2ms
rise time 10~100µs
1.7.11 Baseline Recovery
< 3 s After defibrillation.
1.7.12 Signal Range
± 5 mV p-p
1.7.13 Bandwidth
Surgery 1 ~ 20 Hz
Monitor 0.5 ~ 40 Hz
Diagnostic 0.05 ~ 130 Hz
1.7.14 Calibration Signal
1 mV p-p, ± 5% accuracy
1.7.15 ST measurement
range -2.0 ~ +2.0 mV
Accuracy -0.8mV~+0.8mV: ±0.02mV or ±10%, which is greater
Other range: unspecified
1.8 Respiration
1.8.1 Method
Impedance between RA-LL or RA-LA
1.8.2 Differential Input Impedance
>2.5 M ohm
1.8.3 Respiration Impedance Range
0.3~3Ω
1.8.4 Base Impedance Range
200Ω-2000Ω
1.8.5 Bandwidth
0.3 ~ 2 Hz
1.8.6 Gain
´0.25,´0.500,´1,´2,´4
1.8.7 Respiration Rate
Measurement Range
Adult 0 ~ 120 BrPM
Neonatal / Pediatric 0 ~ 150 BrPM
Resolution 1 BrPM
Accuracy 0~6 BrPM: unspecified
7~150 BrPM: ±2 BrPM or ±2%, use the greater
Inspiriting current < 300 µA RMS max
1.8.8 Apnea Alarm
10 ~ 40 s
1.9 NIBP
1.9.1 Method
Oscillometry
1.9.2 Measure mode
Manual, Auto, STAT
1.9.3 Measure Interval in AUTO Mode
1,2,3,4,5,10,15,30,60,90,120,180,240,480 min
1.9.4 Measure Period in STAT Mode
5 min
1.9.5 Pulse Rate Range
40 ~ 240 bpm
1.9.6 Measure and Alarm Range
Adult Mode
SYS 40 ~ 280 mmHg
DIA 10 ~ 220 mmHg
MEAN 20 ~ 240 mmHg
Pediatric Mode
SYS 40 ~ 220 mmHg
DIA 10 ~ 160 mmHg
MEAN 20 ~ 170 mmHg
Neonatal Mode
SYS 35 ~ 135 mmHg
DIA 10 ~ 100 mmHg
MEAN 20 ~ 110 mmHg
1.9.7 Static pressure accuracy
±3mmHg
1.9.8 Resolution
1mmHg
1.9.9 Accuracy
Maximum Mean error ±5mmHg
Maximum Standard deviation 8mmHg
1.9.10 Overpressure Protection
Adult 300 mmHg
Pediatric 240 mmHg
Neonatal 150 mmHg

1.1 SpO2
1.1.1 Measurement Range
0 ~ 100 %
1.1.2 Resolution
1 %
1.1.3 Accuracy
70% ~ 100% ±2 %
0% ~ 69% unspecified
1.1.4 Pulse Rate
Measure and Alarm Range 20~250bpm
Resolution 1bpm
Accuracy ±3bpm
1.2 Temperature
Channel 2
Measure and Alarm Range 0 ~ 50 °C
Resolution 0.1°C
Accuracy(no sensor) ± 0.1℃(25℃ – 45℃) ,± 0.2℃(other)
Accuracy(include sensor) ± 0.2℃(32℃ - 42℃)
± 0.3℃(other)
1.3 MASIMO Spo2 (optional)
Measurement range 1 to 100%
Resolution 1%
Accuracy 70 to 100%: ±2% (measured without motion in adult/pediatricmode)
70 to 100%: ±3% (measured with motion in neonate mode)
70 to 100%: ±3% (measured with motion)
0% to 69%: Not specified.
Refreshing rate 1 s
Updated period 8 s, 16 s
Pulse Rate
Measurement range 25 to 240 bpm
Resolution 1 bpm
Accuracy ±3 bpm (measured without motion)
±5 bpm (measured with motion)
Refreshing rate 1 s
Updated period 8 s, 16 s
Low Perfusion
Conditions Pulse amplitude: >0.02%
Light penetration: >5%
SpO2 accuracy ±2%
PR accuracy ±3 bpm
1.4 NELLCOR SpO₂(Optional)
1.4.1 SpO2
Range 0~100%
resolution 1%
accuracy 70~100%:±2%
< 69%:undefined
1.5 IBP(optional)
1.5.1 Sensor
reusable sensors:OHMEDA P23XL or BD,EDWARD compatible
once usage:OHMEDA DT-4812 or BD,EDWARD compatible
excitation voltage:+5Vdc±2%
sensitivity:5uV/V/mmHg
1.5.2 Channels
2 channels
1.5.3 Measuer Range
-50~ 360(mmHg)
1.5.4 Resolution
1mmHg
1.5.5 Accuracy(no sensor)
±2% or ±1mmHg, use the greater
1.5.6 Band Width
Normal mode: DC~40Hz
Smooth mode: DC~12.5Hz
1.6 CO₂(optional)
1.6.1 Measuer Range
0% ~ 13%
1.6.2 Resolution
1 mmHg
1.6.3 Accuracy
±2 mmHg @ < 5.0% CO₂ (at ATPS)
1.6.4 Breath Rate
Breath Rate: 3 - 150 bpm
1.7 Gas (optional)
1.7.1 Measuer Range
CO2 0 - 15 %
N2O 0 - 100 %
HAL, ISO, ENF 0 - 8 %
SEV 0 - 10 %
DES 0 - 22 %
1.7.2 Accuracy
CO2 ± (0.2 %ABS + 2 %REL)
O2 ± (1 %ABS + 2 %REL)
N2O ± (2 %ABS + 2 %REL)
HAL, ISO, ENF ± (0.15 %ABS + 2 %REL)
SEV ± (0.15 %ABS + 2 %REL)
DES ± (0.15 %ABS + 2 %REL)
1.7.3 Breath Rate
Breath Rate: 0 - 150 bpm, ±1bpm
1.8 Touch Screen (optional)
1.8.1 Method
4 wire resistor
1.8.2 size
12.1 inch
1.9 Recorder(optional)
Paper width 48 mm
speed 25/50 mm/s
wave channel 3 channels

 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!